29+ calculate the ph of a buffer
Calculate the pH of 0375 L of a 018 M acetic acid-029 M sodium acetate buffer. The pH is defined through the concentration of text H H ions in the solution.
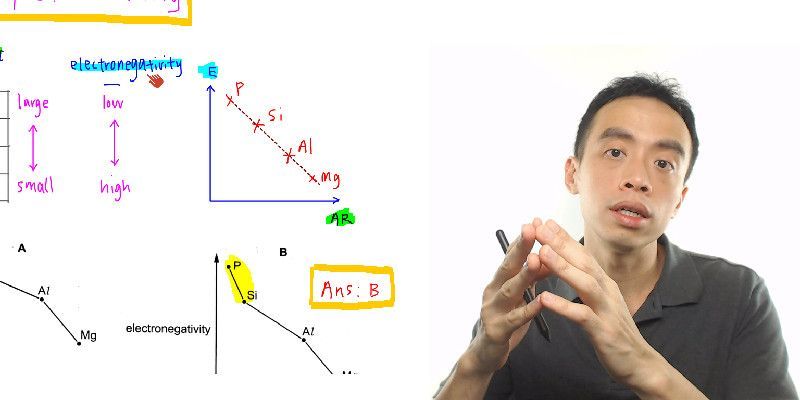
Calculate Ph Of Buffer Solution
First you can use the acid dissociation constant expression and second you can use the Henderson.

. Web So now that were adding the conjugate base to make sure we have roughly equal amounts our pH is no longer 2. Web Chemistry questions and answers. Web You can calculate the pH of buffer solution in two ways.
B Calculate the pH after 10 mL of 010 NaOH is added to 100 mL of. Web Determine the pH of a buffer solution that contains 0100 M hydrazoic acid pKa 460 p K a 460 and 0100 M lithium azide. List the values you are given.
So now we have a strong buffer. Web Calculate the pH of an acetate buffer that is a mixture with 010 M acetic acid and 010 M sodium acetate. Calculate the pH of 0375 L of a 018 M acetic acid-029 M.
Web The pH is a measure of the acidity or basicity of an aqueous solution. Web Answer to Solved Calculate the pH of 930 mL of the buffer solution. Find the pH of a buffer solution that contains.
Web Steps for Calculating the pH of a Buffer Step 1. Web The pH scale is used to measure the acidity or basicity of a solution. It might be somewhere like a 5.
Calculate the pH at 25 of a 0590 M aqueous. Web Calculate the pH of a buffer solution that is 000298 M ethylamine and 000546 M ethylammonium chloride. To determine the pH of the buffer solution we use a.
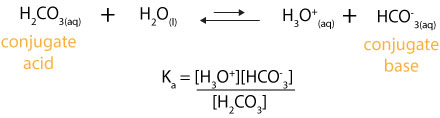
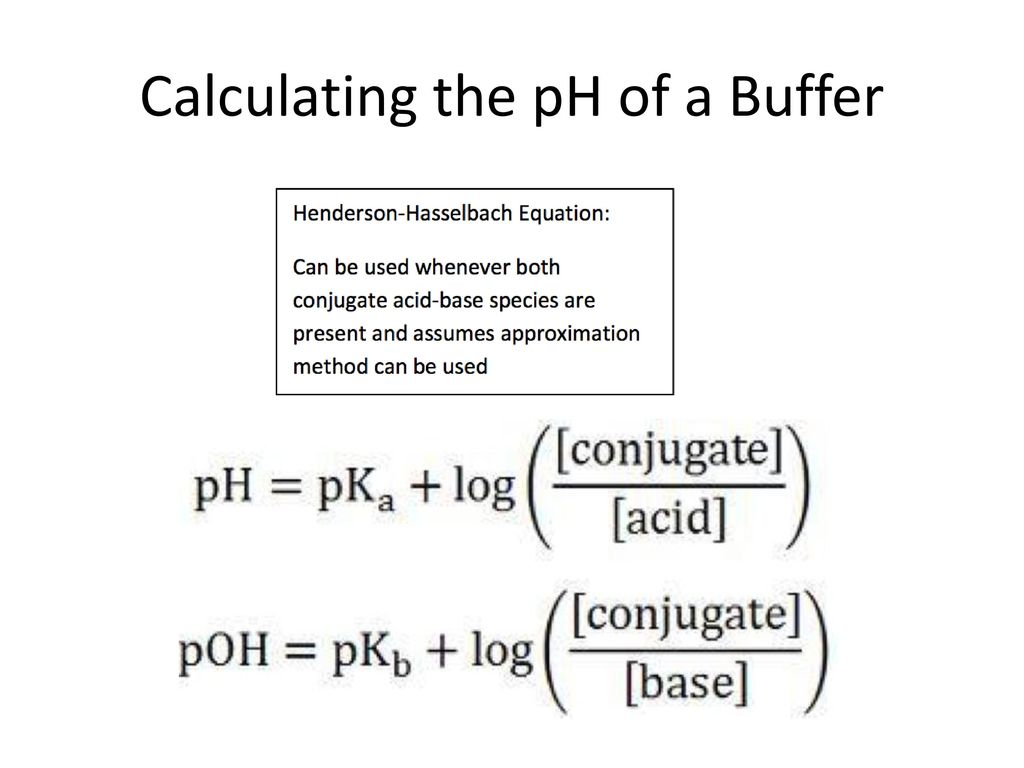
Web One way to determine the pH of a buffer is by using the HendersonHasselbalch equation which is pH pKₐ log A HA. In this equation HA and A refer to the. Web What is the pH of a buffer that results when 632 mole of H3PO4 is mixed with 221 mole of NaOH and diluted with water to 100 L.
Use the values given in the Henderson-Hasselbalch equation to solve for pH. The acid dissociation constants of phosphoric. It ranges from 0 to 14 with 7 being neutral and values above 7 indicating alkaline solutions.
Web Calculate the concentration of the respective constituents in the equilibrium and then proceed with plugging those values into the Henderson-Hasselbalch equation. Web a Calculate the pH of an acetate buffer that is a mixture with 010 M acetic acid and 010 M sodium acetate. PH -log.
Where pKa is the dissociation constant of the acid in this case acetic acid HC2H3O2 A- is the.
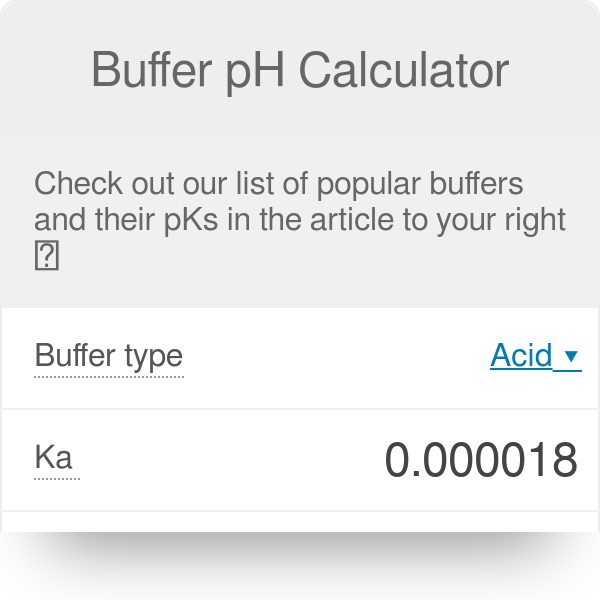
Buffer Ph Calculator
Ijms Free Full Text Complexes Of Cationic Pyridylphenylene Dendrimers With Anionic Liposomes The Role Of Dendrimer Composition In Membrane Structural Changes
How Do You Calculate The Ph Of A Buffer Solution

Solved Time 15m Loulom 0 05m 3uml M T Wrf Nh4cl Hh3 Chegg Com
Buffer Ph Calculations And Buffer Solutions Medium
Buffer Ph Calculations And Buffer Solutions Medium
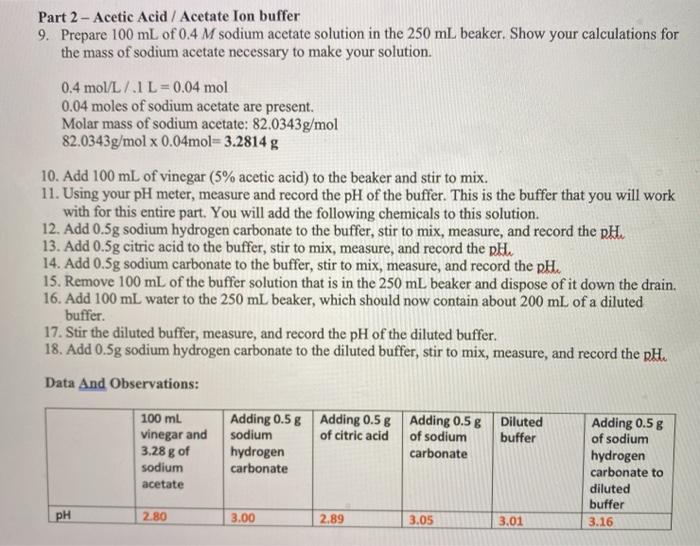
Part 2 Acetic Acid Acetate Ion Buffer 9 Prepare Chegg Com

Part 2 Acetic Acid Acetate Ion Buffer 9 Prepare Chegg Com

Buffers Calculate Ph When A Strong Acid Is Added To Buffer Solution Youtube
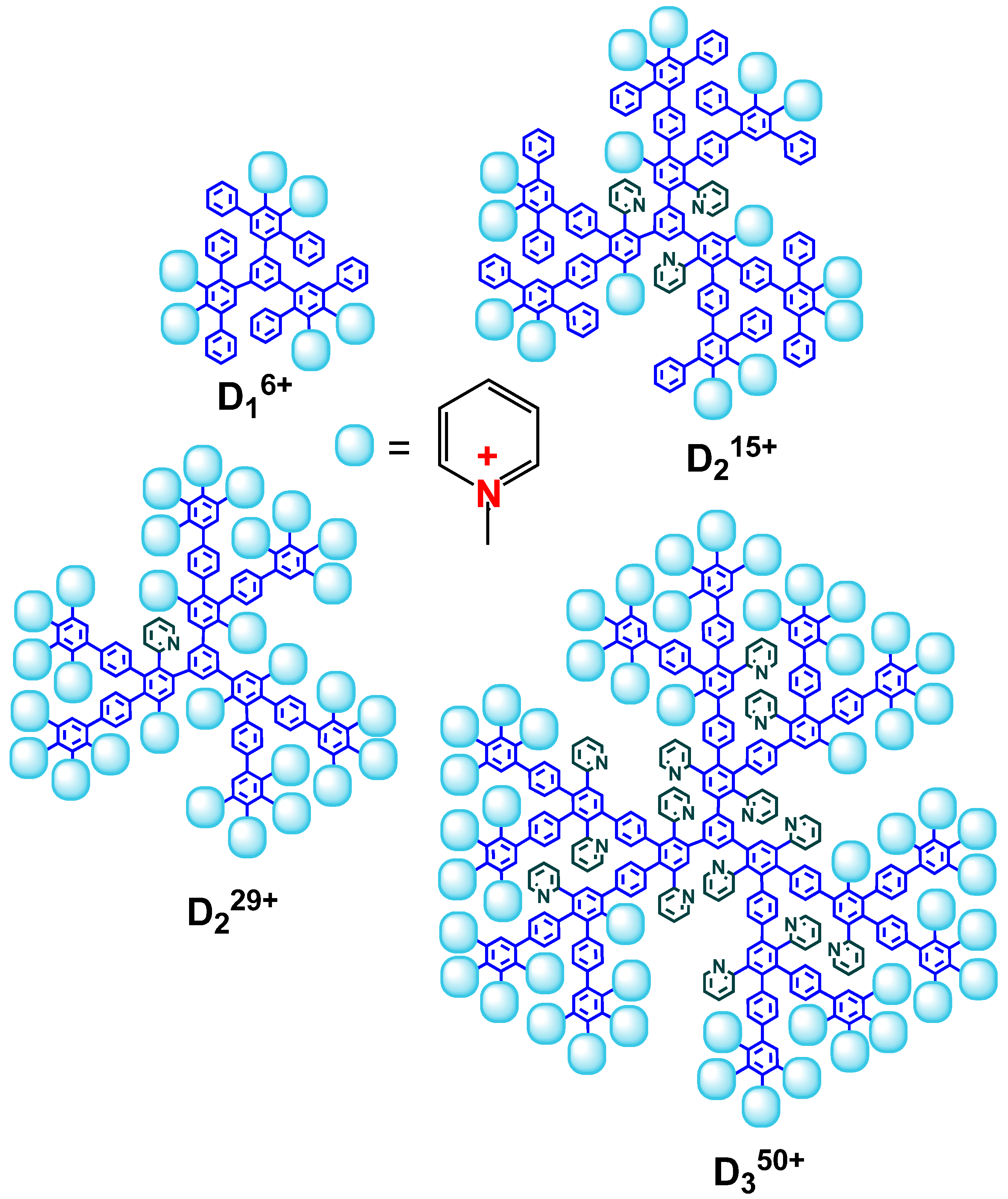
Ijms Free Full Text Complexes Of Cationic Pyridylphenylene Dendrimers With Anionic Liposomes The Role Of Dendrimer Composition In Membrane Structural Changes
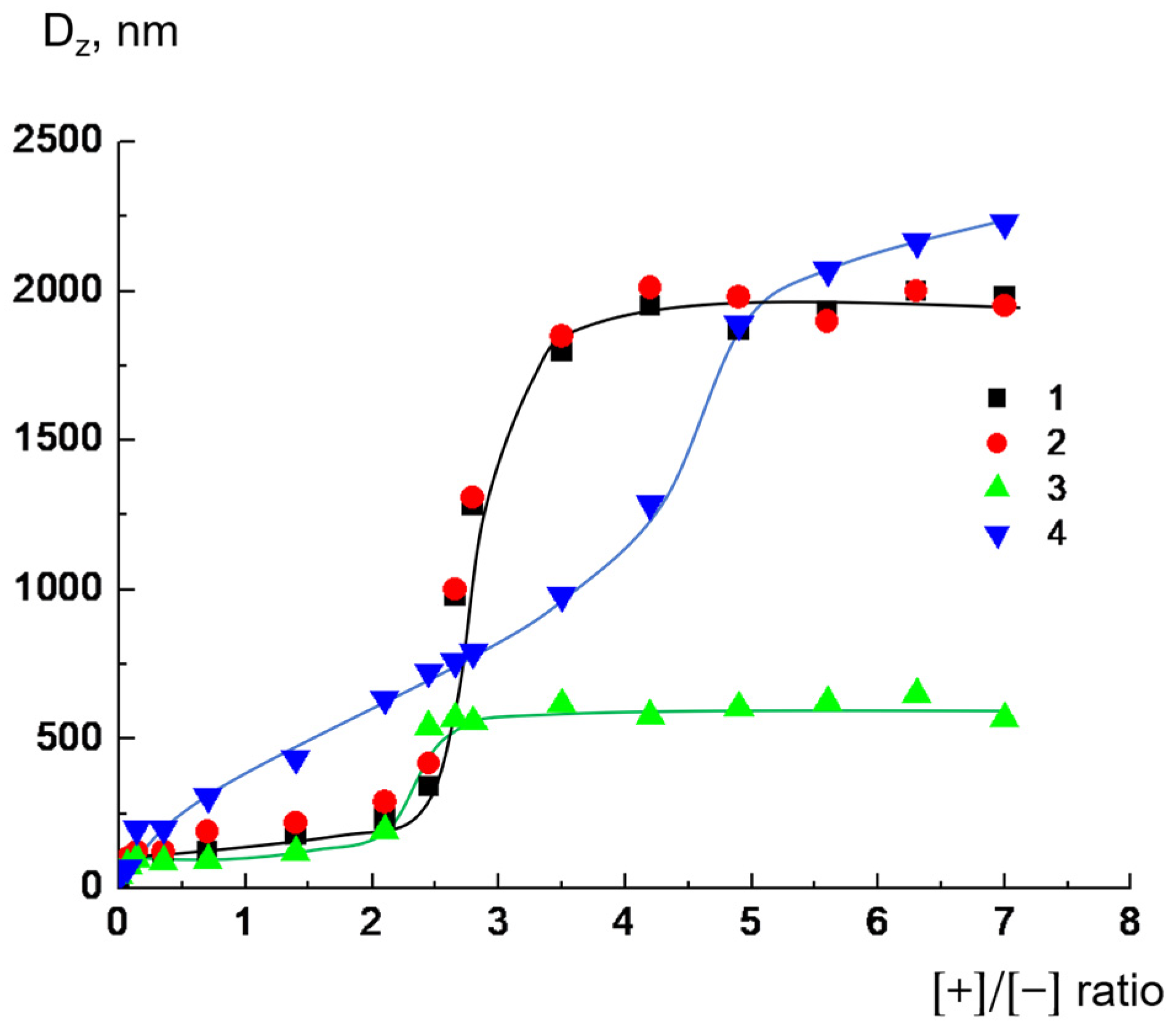
Ijms Free Full Text Complexes Of Cationic Pyridylphenylene Dendrimers With Anionic Liposomes The Role Of Dendrimer Composition In Membrane Structural Changes

Part 2 Acetic Acid Acetate Ion Buffer 9 Prepare Chegg Com
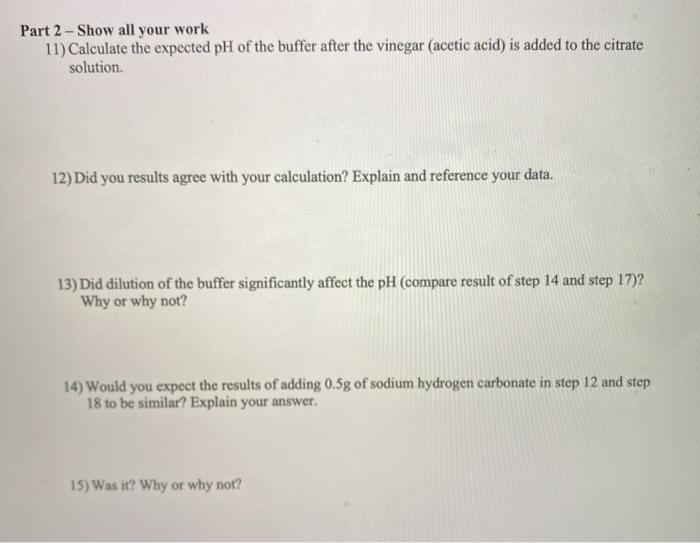
Part 2 Acetic Acid Acetate Ion Buffer 9 Prepare Chegg Com
![]()
Step By Step Approach To Arterial Blood Gas Analysis

How Do You Calculate The Ph Of A Buffer Solution

Calculate Ph Of Buffer Solution
Chapter 8 8 Buffer Systems Ppt Download